What Does Buffer Solution Mean In Biology
I dont knw the meaning of 1x and 10x please explain me. Buffers are solutions that resist a change in pH on dilution or on addition of small amounts of acids or alkali.
 Buffer Solutions Biochemistry The Biology Notes
Buffer Solutions Biochemistry The Biology Notes
Stronger bases B are also changed into weak acids BH with the rise in HCO 3.

What does buffer solution mean in biology. It is a water-based salt solution containing disodium hydrogen phosphate sodium chloride and in some formulations potassium chloride and potassium dihydrogen phosphate. A capacity of 1 when 1 mol of acid or alkali is added to 1 litre causes a pH fall or rise of 1 pH unit. Its pH changes very little when a small amount of strong acid or base is added to it and thus it is used to prevent changes in the pH of a solution.
A buffer consists of a weak acid and its conjugate base or a weak base and its conjugate acid. A buffer solution is one which resists changes in pH when small quantities of an acid or an alkali are added to it. It serves to maintain the correct pH necessary as many enzymes work only under precise pH conditions.
The driving force that separates proteins in a gel is an electric field. Return to Search Page. A buffers pH changes very little when a small amount of strong acid or base is added to it.
Prepare 1 liter of 1X TBE buffer from a 10X TBE stock solution. In biochemistry a buffer specifically a buffer solution is essential for many biochemical processes. The bicarbonate buffer neutralizes stronger dietary and metabolic acids HA converting them into weak bases A with the increase in H 2 CO 3.
Phosphate-buffered saline abbreviated PBS is a buffer solution commonly used in biological research. A buffer is an aqueous solution used to keep the pH of a solution nearly constant. Buffers are extremely useful in these systems to maintain the pH at a constant value.
To produce the field both ends of a gel and respective positive and negative electrodes are immersed in a solution that we call electrode buffer and a typical class goes through 30 liters or so of the stuff. It is used to prevent any change in the pH of a solution regardless of solute. Buffer capacity is the amount of acid or base that can be added before the pH of a buffer changes.
This does not mean that the pH of buffers does not change. A buffer is an aqueous solution consisting of a mixture of a weak acid its salt or a weak base its salt that resist a change in pH on the addition of either acid or base. From obsolete buff to make a sound like a soft body being hit of imitative origin.
A buffer is a mixture of an acid that does not ionize completely in water and its corresponding base-for example carbonic acid H 2 CO 3 and sodium bicarbonate NaHCO 3. In order to save time and space molecular biologists often make concentrated stocks of solutions to last over long periods of time. Such concentrated stocks take up less space.
Buffer solution A buffer is an aqueous solution consisting of a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid. Note- A lot of biological chemical reactions need a constant pH for the reaction to proceed. Buffers typically consist of an acid-base pair with the acid and base differing by the presence or absence of a proton a conjugate acid-base pair.
In a molecular biology research lab you will constantly need to make and use buffers. For example the bicarbonate buffering system is used to regulate the pH of blood. An example of a buffer solution is bicarbonate in blood which maintains the bodys internal pH.
They help in a neutralization reaction to a certain extent. Buffers are important in biological systems because of their ability to maintain constant pH conditions. A lot of biological and chemical reactions need a constant pH for the reaction to proceed.
For instance one of the buffers that maintain the pH of human blood involves carbonic acid H. Buffer capacity A measure of the ability of a solution to maintain its pH in the face of the addition of acid or alkali. In nature there are many systems that use buffering for pH regulation.
A buffer is an aqueous solution containing a weak acid and its conjugate base or a weak base and its conjugate acid. In lab the buffer solution or PBS solution is made in 1x or 10x concentration. Buffers are solutions that moderate pH changes when an acid or base is added to the buffer system.
Its pH changes very little when a small amount of strong acid or base is added to it. A buffer solution is an aqueous solution consisting of a mixture of a weak acid and its conjugate base or vice versa. Buffer solutions are solutions in water that mark the combination of acids and bases.
What is the Structure of Nephron and its Functions. Acidic buffer solutions are those that have strong acids and weak bases as their components. If acid is added to this buffer the added H ions combine with bicarbonate ions to produce more carbonic acid using up some of the H ions the Na ions do not participate in this reaction.
The buffer helps to maintain a constant pH. Buffer solutions are used as a means of keeping pH at a nearly constant value in a wide variety of chemical applications.
 Buffer Solution Its Characteristics Types And Preparations
Buffer Solution Its Characteristics Types And Preparations
 A A Diagram Of The Process Of Agarose Gel Electrophoresis 1 An Agarose And Buffer Solution Is Heated And Poured Microbiology Study Chemistry Study Biology
A A Diagram Of The Process Of Agarose Gel Electrophoresis 1 An Agarose And Buffer Solution Is Heated And Poured Microbiology Study Chemistry Study Biology
 Ways To Get A Buffer Solution Video Khan Academy
Ways To Get A Buffer Solution Video Khan Academy
 Buffer Solution Acidic Buffer Basic Buffer Animation Buffer Solution Electron Configuration Solutions
Buffer Solution Acidic Buffer Basic Buffer Animation Buffer Solution Electron Configuration Solutions
 Buffer Solutions Buffer Solution Fun Learning Ap Chemistry
Buffer Solutions Buffer Solution Fun Learning Ap Chemistry
 Buffer Solution Preparation Of Buffer Solution Acidic Basic Buffer Buffer Action Buffer Solution Electron Configuration Solutions
Buffer Solution Preparation Of Buffer Solution Acidic Basic Buffer Buffer Action Buffer Solution Electron Configuration Solutions
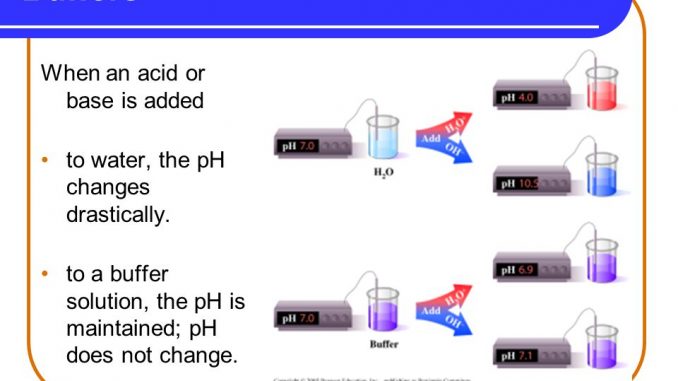 Buffer Buffering Capacity Properties Of Good Buffer And Role Of Buffer In Vitro And In Vivo Online Biology Notes
Buffer Buffering Capacity Properties Of Good Buffer And Role Of Buffer In Vitro And In Vivo Online Biology Notes
 Buffer Solution Preparation Of Buffer Solution Acidic Basic Buffer Buffer Action Buffer Solution Solutions Electron Configuration
Buffer Solution Preparation Of Buffer Solution Acidic Basic Buffer Buffer Action Buffer Solution Solutions Electron Configuration
 Buffer Solutions Chemistry Review Buffer Solution Chemistry
Buffer Solutions Chemistry Review Buffer Solution Chemistry
 How To Prepare Buffer Solutions Buffer Solution Teaching Chemistry Chemistry Jokes
How To Prepare Buffer Solutions Buffer Solution Teaching Chemistry Chemistry Jokes
 Buffer Solution Ph Calculations Henderson Hasselbalch Equation Explained Chemistry Problems Youtub Teaching Chemistry Science Chemistry Biochemistry Notes
Buffer Solution Ph Calculations Henderson Hasselbalch Equation Explained Chemistry Problems Youtub Teaching Chemistry Science Chemistry Biochemistry Notes
 Buffer Solution Ph Calculations Video Khan Academy
Buffer Solution Ph Calculations Video Khan Academy
 Buffers Definition Overview Expii
Buffers Definition Overview Expii




